A Typical Vitamin C Tablet Weighs 500 Mg
Typical vitamin C tablet (containing pure ascorbic acid, H2C6H606) weighs 500 mg: One vitamin C tablet is dissolved in enough water to make 190.0 mL of solution_ Calculate the pH of this solution Ascorbic acid is a diprotic acid. For HzC6H6O6 Kal = 7.9*10-5 and Ka2 L.6x10-12pHSubmit AnswerTry Another Verslonitem attempts remaining
typical vitamin C tablet (containing pure ascorbic acid, H2C6H606) weighs 500 mg: One vitamin C tablet is dissolved in enough water to make 190.0 mL of solution_ Calculate the pH of this solution Ascorbic acid is a diprotic acid. For HzC6H6O6 Kal = 7.9*10-5 and Ka2 L.6x10-12 pH Submit Answer Try Another Verslon item attempts remaining

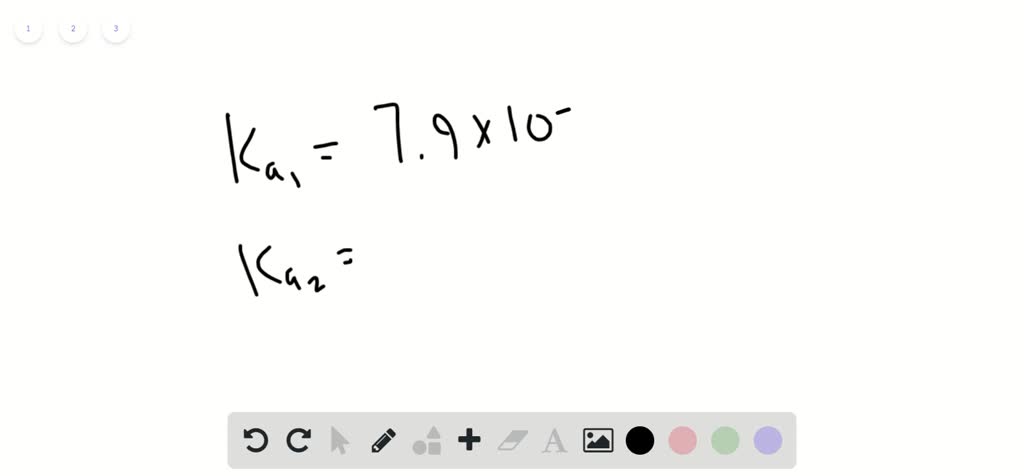
A typical vitamin $\mathrm{C}$ tablet (containing pure ascorbic acid, $\mathrm{H}_{2} \mathrm{C}_{6} \mathrm{H}_{6} \mathrm{O}_{6}$ ) weighs $500 . \mathrm{mg}$ . One vitamin $\mathrm{C}$ tablet is dissolved in enough water to make 200.0 $\mathrm{mL}$ of solution. Calculate the $\mathrm{pH}$ of this solution. Ascorbic acid is a diprotic acid.
This problem. We have a Scorpio kassid C six h a 06 But because I don't want to write this out every time I'm gonna simplify its H. In other words, just some generic acid. And so this reaction is going to be h A plus water being an equilibrium with a minus and h 20 plus. And so, as always, this sort of problem is going to be a nice people problem with initial change and equilibrium, This equilibrium site problem. This is always a case of acid base problems. So it's work out. What we're given were given that we have 0.25 milligrams, which is or 250 milligrams which is equal 2.25 grams in ah, 0.25 leaders. And so we want to get this in Mill Araji. And so the way we're gonna do that is wearing divide 0.25 grams by the molecular weight, which is equal to 1 76.12 grams per mole. And that's going to give us the amount of moles of C six h eight. And so I'll tell you, that is, um, one point for one times 10 to the negative third moles. And so then, if we divide that by 0.25 will get the mill Araji, and that's going to be equal to 5.67 times 10 to negative third more. This is really not all acid based cancer. This is so calm. A tree changing from grams of grams per litre, toe moles per liter. But this is gonna be our initial concentration of a chain and saw blood that is 5.6 times sensitive. Third, ignore water 00 as our initial conversations there, minus X plus ax plus actions going through the process of you doing a nice table. So this is what happens when you add up the previous soup bones. And so remember, we're gonna write out the mass action expression K is equal to the concentration of a minus times conservation of HBO, plus over the concentration of H A, which plugging in we have right here acts, acts so that goes right there and right there X squared over 5.6 times Centenary of third minus X now were given K for this. So I'll plug it in right here. It's eight times tense any of fifth. And so that tells us is that we can if we solve for X, we can get the concentration HBO plus and then throughout the pH. Now, this accident here is much smaller than that. Um, normally speaking, Tencent interferes a pretty small number, so it might not be a completely safe assumption. So if you're really being careful, I would plug it, keep it there and use probably like the club Jack equation, or used a graphic tap to figure it out most of time. We just ignore mind that minus X there. And either way we're gonna get a value for X, which is equal to 6.7 time sense. And I get 1/4 Moeller, which is equal to the concentration of H 20 plus from the ice table. That's how we know it. And remember, the negative log of the conservation H three or plus equals the pH, which is what we're being asked for in the pH. When you do that is 3.2
A score back acid is a dye product. Weak acid with the chemical formula h two C six each, 606 And for us to find the pH of a 0.250 Mueller solution. Let's start with the first ionization each to see six, 8606 h 20 in equilibrium is it is a weak acid. We will get each 30 plus and each c six h 606 minus a Christs. We're gonna create a nice table for the weak acid, starting with 0.250 Moeller. It's completely Rice Table Ridder. Kiai. One expression here we can substitute our values in K won for a score. Big acid. We may look up and it's equal to 7.9 times 10 to the negative five substitute our values in which will yield a quadratic equation. Let's go ahead and sold for this quadratic equation. Not going to go through the steps for solving this. You can go ahead and verify that your answer is going to match up with, uh, my ad, sir, so X squared and we're going to have 0.250 minus X and the denominator solving will yield two routes. The first street is 0.44 and negative 0.45 We're going to reject the negative roots and we see that excess 0.44 Therefore, uh, the polarity of the H 30 plus. It is also equal to the military t of the n i n. Those are both equal 2.0 for four Moeller. So there's the first ionization, the second ionization. All right, the brown said Lowry equations for the second ionization. Great nice table reserve for four winters are for four zero Kalita race table and were there k A to expression here, products over react INTs substitute our values. Now we're gonna look up the key a two for ascorbic acid, which is equal to 1.6 times 10 to the negative 12 substitute or values in again. We here going to have a quadratic equation which we're going to go ahead and solved. And you can also verify yourself that the roots that you get are that seem, is what I get. So the two routes they end up with here are going to be zero and negative 0.0 440 I'm gonna re, uh, redo this here, Teoh. Maybe get this a little bit more precise. Let's see if I can get my roots here. And the roots are We're going. It's such a small value due to the small KB that it does work out to be essentially zero. There would be more to it, um, than that we're going to reject the negative root I have my equations over is not giving me any anything more than zero there, so really O h minus after the 2nd 1 here is equal to zero. So the total ohh minus is only due to the first ionization here. Sorry, The each 30 plus, due to the second ization ionization here is zero. The total age three or plus, is only due to the first ionization, which is 0.44 Moeller and then the pH here would be negative. Log 0.44 again work. Taking that second ionization to be negligible as it does work out to zero in accordance, or sigfig. So our ph here would be a value that's equal to 2.36
So let's do a special type of acid base reaction via tit tray shin titillation is a very accurate way of measuring substances. And so we have a reaction here between vitamin C. And sodium hydroxide. And um let's try to figure out you know, vitamin C. People normally take uh take a pill and uh let's see if there might be any impurities in the vitamin C. Now when we do this reaction, what we're gonna know is um that we needed um 21.3 ml of sodium hydroxide. And the polarity of that was .1250 to react the all of the vitamin C. And we know that we have a 500 mg tablet, vitamin C. So let's figure out Um how much of that 500 mg tablet was vitamin C. And then whatever the differences must be, whatever the impurities are. So um let's find the moles of sodium hydroxide. Then we can find the moles of the vitamin C. And then we can find the mass of the vitamin C. So we'll start out with our 21.30 ml. Uh we need to get that to leaders in order to use more clarity. So every leader is 1000. Remember polarity is moles per liter. So we have a .1250 mol per leader solution of the sodium hydroxide. So if we stopped here, that would get us moles of sodium hydroxide. But I want to know about the vitamin C tablets. So I'm gonna need the mole ratio. And what we do know is that we have one mole of our vitamin C. For every mole of sodium hydroxide we use. And so to get that two g to figure out how much of it we have, We can multiply by the molar mass of the Vitamin C, which is 176.12 g and the tablet is in milligrams. So we might as well convert two mg. Now there's 1000 milligrams anagram. And that's going to get us the grams of our vitamin C, 468 0.6 mg of our vitamin C. Yeah, but we have a 500 mg tablet. Well 468.6 of that is vitamin C. The balance must be the impurities. So the impurity, it's going to be the total. Uh that's milligrams. There are 500 mg- R. 4 68.6 mg of are known Vitamin C. And so we have 31 four g of the impurity. Okay, And just for significant figures, I should have put in an extra zero there because this actually is a 500 mg tablet. So to find the percent impurity, we're going to take the amount of impurity over the total And multiply it by a 100 and 6.28 is the percentage of the impurities in our tablet.
In this question. It is given esque or big sec 6 82 6 is a die protic acid. And we have to find the ph of the solution which contains five mg ascorbic acid par militar of water. And here we have to assume only first ionization is important for determining the peach. And here key A. One is also given as 7.9 times 10 to the power negative five. No, this first considered the concentration of the acid and the in the solution it is five mg acid, para millimeter of water. That means five g acid barley debt solution. Now the molecular mask of this S Corby gassy is 176 g bar moon. So the Mueller concentration of this solution, Mueller concentration of this solution is five divided by 1 76. That will be the more part lita. And the value is 010- eight More Park. Later that means 0.028 molar. There's the concentration of that as its solution Now decide the organization equation for this acid C68846 blast water. It produces C. 68706 minus plus ace trio plus. Let's make that I step on here. Initial concentration 0.28 00. Change concentration minus six plus six plus X. And the equilibrium concentration 0.28 minus six X. And X. Let's like that expression for K. A. K. A. His concentration of c.- Our times a studio plus and divided by the concentration of c. All six. That's the key. A. Now K. Here is 7.9 times 10 to the power negative five. And the concentration of C. C. C. 706 minus is equal to concentration of his three A. Plus both eggs or right X. Square divided by equilibrium concentration of the C. 0.28 minus eight. Here we can make the simplifying assumption because if the concentration of the acid is greater than K. Eight times sunday here Kay is 7.19 to the power minus five. If we multiply by 100 it will be 7.9 into the power minus three. And concentration of the sed 0.28. So obviously concentration of sed is greater than 100 times K. So we can make this simplifying assumption. So x. 79 times 10 to the 4 -5 Time 0.028. So eggs will be square root top 7.19 to depart -5 times 0.028. So the value for X is 2.0 times 10 to the power uh minus. Yeah. No not this one X. Is 1.41 times 10 to the four minus three a.m. This is the concentration of high dhoni um Iron. So this is 1.41 times 10 to the four negative three. And so ph is negative log Adonia man concentration 1.41 time straight to the bar negative three, So ph d s 2.85 Yeah.
5 answers
Suppose that the functions 'and g are defined as follows. fk} V3x+4 gk) =-4r2+5Find f+g and f: g Then, give their domains using Interval notation.6 +2)6) =6 (O,D) [,E]Domain of f + g : 0DUD (O,2] [,D)C0~CO6*8)6) = 092Domaln of f* g
Suppose that the functions 'and g are defined as follows. fk} V3x+4 gk) =-4r2+5 Find f+g and f: g Then, give their domains using Interval notation. 6 +2)6) = 6 (O,D) [,E] Domain of f + g : 0 DUD (O,2] [,D) C0 ~CO 6*8)6) = 0 9 2 Domaln of f* g...
5 answers
18. Consider the line with vector equation (x,y,=) = (3,4,-1) +0(1,-2,5) and the point P(2,5,7) Show that P is not on L, and then find a Cartesian equation for the plane that contains both P and L.
18. Consider the line with vector equation (x,y,=) = (3,4,-1) +0(1,-2,5) and the point P(2,5,7) Show that P is not on L, and then find a Cartesian equation for the plane that contains both P and L....
A Typical Vitamin C Tablet Weighs 500 Mg
Source: https://itprospt.com/num/1265735/typical-vitamin-c-tablet-containing-pure-ascorbic-acid-h2c6h606

0 Komentar